- Information Technology
- Data Infrastructure
- Data Tools
- Desktops, Laptops and OS
- Chip Sets
- Collaboration Tools
- Desktop Systems - PCs
- Email Client
- Embedded Systems
- Hardware and Periferals
- Laptops
- Linux - Open Source
- Mac OS
- Memory Components
- Mobile Devices
- Presentation Software
- Processors
- Spreadsheets
- Thin Clients
- Upgrades and Migration
- Windows 7
- Windows Vista
- Windows XP
- Word Processing
- Workstations
- Enterprise Applications
- IT Infrastructure
- IT Management
- Networking and Communications
- Bluetooth
- DSL
- GPS
- GSM
- Industry Standard Protocols
- LAN - WAN
- Management
- Mobile - Wireless Communications
- Network
- Network Administration
- Network Design
- Network Disaster Recovery
- Network Interface Cards
- Network Operating Systems
- PBX
- RFID
- Scalability
- TCP - IP
- Telecom Hardware
- Telecom Regulation
- Telecom Services
- Telephony Architecture
- Unified Communications
- VPNs
- VoIP - IP Telephony
- Voice Mail
- WAP
- Wi-Fi (802.11)
- WiMAX (802.16)
- Wide Area Networks (WAN)
- Wireless Internet
- Wireless LAN
- Security
- Servers and Server OS
- Software and Web Development
- .Net Framework
- ASPs
- Application Development
- Application Servers
- Collaboration
- Component-Based
- Content Management
- E-Commerce - E-Business
- Enterprise Applications
- HTML
- IM
- IP Technologies
- Integration
- Internet
- Intranet
- J2EE
- Java
- Middleware
- Open Source
- Programming Languages
- Quality Assurance
- SAAS
- Service-Oriented Architecture (SOA)
- Software Engineering
- Software and Development
- Web Design
- Web Design and Development
- Web Development and Technology
- XML
- Storage
- Agriculture
- Automotive
- Career
- Construction
- Education
- Engineering
- Broadcast Engineering
- Chemical
- Civil and Environmental
- Control Engineering
- Design Engineering
- Electrical Engineering
- GIS
- General Engineering
- Industrial Engineering
- Manufacturing Engineering
- Materials Science
- Mechanical Engineering
- Medical Devices
- Photonics
- Power Engineering
- Process Engineering
- Test and Measurement
- Finance
- Food and Beverage
- Government
- Healthcare and Medical
- Human Resources
- Information Technology
- Data Infrastructure
- Data Tools
- Desktops, Laptops and OS
- Chip Sets
- Collaboration Tools
- Desktop Systems - PCs
- Email Client
- Embedded Systems
- Hardware and Periferals
- Laptops
- Linux - Open Source
- Mac OS
- Memory Components
- Mobile Devices
- Presentation Software
- Processors
- Spreadsheets
- Thin Clients
- Upgrades and Migration
- Windows 7
- Windows Vista
- Windows XP
- Word Processing
- Workstations
- Enterprise Applications
- IT Infrastructure
- IT Management
- Networking and Communications
- Bluetooth
- DSL
- GPS
- GSM
- Industry Standard Protocols
- LAN - WAN
- Management
- Mobile - Wireless Communications
- Network
- Network Administration
- Network Design
- Network Disaster Recovery
- Network Interface Cards
- Network Operating Systems
- PBX
- RFID
- Scalability
- TCP - IP
- Telecom Hardware
- Telecom Regulation
- Telecom Services
- Telephony Architecture
- Unified Communications
- VPNs
- VoIP - IP Telephony
- Voice Mail
- WAP
- Wi-Fi (802.11)
- WiMAX (802.16)
- Wide Area Networks (WAN)
- Wireless Internet
- Wireless LAN
- Security
- Servers and Server OS
- Software and Web Development
- .Net Framework
- ASPs
- Application Development
- Application Servers
- Collaboration
- Component-Based
- Content Management
- E-Commerce - E-Business
- Enterprise Applications
- HTML
- IM
- IP Technologies
- Integration
- Internet
- Intranet
- J2EE
- Java
- Middleware
- Open Source
- Programming Languages
- Quality Assurance
- SAAS
- Service-Oriented Architecture (SOA)
- Software Engineering
- Software and Development
- Web Design
- Web Design and Development
- Web Development and Technology
- XML
- Storage
- Life Sciences
- Lifestyle
- Management
- Manufacturing
- Marketing
- Meetings and Travel
- Multimedia
- Operations
- Retail
- Sales
- Trade/Professional Services
- Utility and Energy
- View All Topics
Share Your Content with Us
on TradePub.com for readers like you. LEARN MORE
Request Your Free Playbook Now:
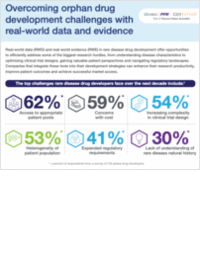
"Ensuring the biosafety of advanced therapies with NGS (Next-Generation Sequencing)"
This playbook explores biologics safety, regulatory compliance, NGS innovations, virus detection, and advanced therapy quality assurance.
Biologics and cell- and gene-based therapies are already benefitting many patients around the globe. But for innovative new treatments to reach their potential to improve patient lives they must be proven safe and adhere to a variety of regulations.
Biologics and cell- and gene-based therapies pose the risk of contamination because they are manufactured using viral vectors, genetic material, and living cells. Fortunately, innovations in next-generation sequencing (NGS) are evolving quickly to help ensure the safety of advanced therapies. Download this playbook to understand:
- How regulations such as CFR 21 Part 11 and ICH Q5A(R2) apply to biologics and cell- and gene-based therapies.
- The limits of polymerase chain reaction (PCR) and cytopathic effect (CPE) tests to detect adventitious viruses in biologics and cell- and gene-based therapies
- The efficiency and flexibility of NGS-based approaches to adventitious virus detection.
Offered Free by: BioPharma Dive's studioID and Qiagen
See All Resources from: BioPharma Dive's studioID and Qiagen